CAUSAL ROLE OF BETA BURSTS IN INHIBITORY CONTROL OF MOVEMENT
PIs
Prof. Dr. Ilka Diester
Prof. Dr. Tonio Ball
Summary
The ability to withhold a response and appropriately time one’s actions is an essential component of goal directed behavior. Such cognitive control is exercised through the action of dedicated circuits, large part of which is situated in the prefrontal cortex (PFC) (1). Medial prefrontal cortex (mPFC) in rats is a known site of delay-related activity during performance of a simple reaction time task; and its subsections, the prelimbic and infralimbic cortices differentially affect the animal’s ability to respond (2). How does mPFC integrate and communicate relevant information to maintain control over responses - remains largely unclear.
One candidate mechanism are cortical oscillations – fluctuations of extracellular potential, also termed local field potentials (LFP). Cortical oscillators provide a natural way to assemble remote neural circuits into functional ensembles whose activity can guide appropriate, task-relevant responses (3). Although LFP activity is broadband, consisting of all fluctuations below ~300Hz, a particular spectral subset of this activity in the PFC - beta bursts (Fig. 1A) – is persistently shown to correlate with successful response inhibition in humans (4–6). Previously, using well established methods, we have detected beta burst events across the subregions of the mPFC in rats, while they performed a rodent version of the response inhibition task. These were obtained from multi-laminar recordings using silicon probes. General analysis of bursting events yielded a statistical description consistent with our lab’s previous report of similar events in the primary sensory cortex (7). In addition, we observed a robust association of animal performance and beta burst rate in the prelimbic subregion (Fig. 1B). Briefly, bursting was increased during periods of lever pressing, and it gradually decreased as the trial progressed, which is especially evident during the correct long trials. Most notably, there was a significant difference between baseline burst rates of correct and early trials, as bursting was decreased on average during trials when the animal made an incorrect, early lever release. The observations also support our previous findings in the prelimbic cortex, the activity of which was established as crucial for successful inhibition (2).
This starts to suggest a link between the prefrontal beta and a previously postulated mechanism of response control, in which the mPFC establishes a firm inhibition over the motor system and allows appropriate preparation and execution of movement. This is the main idea of the current proposal - to further investigate this association for its possible causal role. We suggest to use a closed-loop system for dynamic on-line modulation of task parameters during the response inhibition task (Fig. 1C). As described previously, LFPs and single/-multi-unit activity will be recorded using multi-shank, laminar silicone probes, spanning the 2 major areas of the mPFC. As the current hypothesis states that beta activity in the prelimbic region predicts successful inhibition, beta events would be used to infer the state of behavioral inhibition and then correspondingly adjust the ongoing task demands, mainly the length of the delay period. Alternatively, persistent (spanning multiple trials) states of high/low beta would be used to adjust the trial block structure, i.e. the frequency of long and short trials within larger blocks of trials. The animal’s ability to answer these changing demands compared to control conditions would therefore be the main readout, and offer strong support for direct causality. Finally, individual beta events would be tested for their effect on population activity within the mPFC. Any event causal for the system under study, should provide detectable constraints on the surrounding network, most strongly evidenced by concurrent changes in the firing properties of constituent neurons. Previously, we have successfully demonstrated the utility of this real-time system in a similar paradigm, but different cortical system (8). Although the median length of beta bursts is around 120ms, our system allows us temporally accurate detection on a very similar timescale.
As described in the Fig. 1A caption, beta bursts can be decomposed into multiple features, each of which potentially encodes task-relevant information (4). To enable more accurate on-line feedback, and eliminate inherent bias towards envelope-instructed burst definition in our closed-loop system, we propose to implement deep convolutional networks (ConvNets) which show remarkable potential and state-of-the-art level performance in decoding electroencephalographic data (9). ConvNets would be used for off-line data analysis and decoding of trials based on either pre-defined or independently learned sets of beta-band features. Of course, the approach can be generalized to a minimally-filtered signal, as to incorporate features present across multiple spectral bands. Finally, the collaboration enables us to apply the beta burst extraction algorithms on human data coming from different motor tasks. We expect to detect similar activity signatures in these datasets, and suggest to use beta-derived features to improve the decoding accuracy of current algorithms.
In addition to their causal role, the origin of beta oscillations is also a topic of great interest and debate. We aim to chronically record large-scale activity across the midfrontal regions of the cortex using ECoG arrays. This will provide us with a much-needed insight into the dynamics of beta bursts across the motor system during task performance, the coherence of the participating structures and their position within the cortical beta hierarchy. This will enable important comparisons with a much greater human literature on this subject. Beta oscillations in the motor circuits are best known for their abnormal increase in the basal ganglia system of Parkinson’s disease patients. This subcortical network will be at the focus of our future attention, especially the structures immediately downstream of the mPFC – striatum and subthalamic nucleus, as the most likely targets of direct executive control of the mPFC, but any questions in this regard, are predicated upon further supporting data from the experiments described in this proposal.
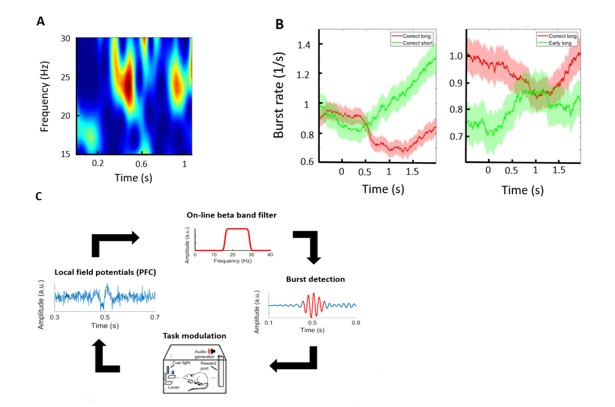
Figure 1: Proposal summary. A: An example of multiple beta bursts recorded within a 1s time window represented as a spectrogram. Beta bursts are discrete, brief increases in the amplitude of local field potentials spanning the 15-30Hz frequency range. The color in the spectrogram represents the power (amplitude squared) at each time point and frequency. Detection of bursts therefore enables extraction of various statistical parameters such as burst rate (number of events per second, similar to λ, the main parameter of the Poisson distribution), frequency (central frequency of individual events), amplitude envelope, inter-burst interval (temporal separation of events, related to rate), etc. B: Mean burst rate across different trials during the performance of a response-inhibition task. The animal was trained to hold a lever for 1s (long) or 300ms (short). and release it in response to a tone. Trials are aligned to the lever press (0s). Diagrams compare the rate between different types of trials when the animal correctly released the lever (left) or between correct and incorrect (early) responses of the same trial type (right). C: The closed-loop behavioral system. LFP is recorded and filtered on-line within the beta band. The power envelope of the filtered signal is then scanned for threshold crossings, indicating significant increases in beta activity. Such detected events are then used to dynamically change the task demands in accordance with the hypothesis.
Related Publication
1. Miller EK. The prefontral cortex and cognitive control. Nat Rev Neurosci. 2000 Oct;1(1):59-65.
2. Hardung S, Epple R, Jäckel Z, Eriksson D, Uran C, Senn V, et al. A Functional Gradient in the Rodent Prefrontal Cortex Supports Behavioral Inhibition. Current Biology. 2017 Feb 20;27(4):549-55.
3. Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015 Oct 7;88(1):220-35.
4. Enz N, Ruddy KL, Rueda-Delgado LM, Whelan R. Volume of β-Bursts, But Not Their Rate, Predicts Successful Response Inhibition. J Neurosci. 2021 Jun 9;41(23):5069-79.
5. Wessel JR. β-Bursts Reveal the Trial-to-Trial Dynamics of Movement Initiation and Cancellation. J Neurosci. 2020 Jan 8;40(2):411-23.
6. Diesburg DA, Greenlee JD, Wessel JR. Cortico-subcortical β burst dynamics underlying movement cancellation in humans. Swann NC, Ivry RB, Muralidharan V, Schmidt R, editors. eLife. 2021 Dec 7;10:e70270.
7. Karvat G, Schneider A, Alyahyay M, Steenbergen F, Tangermann M, Diester I. Real-time detection of neural oscillation bursts allows behaviourally relevant neurofeedback. Communications Biology. 2020 Feb 14;3(1):1-10.
8. Karvat G, Alyahyay M, Diester I. Spontaneous activity competes with externally evoked responses in sensory cortex. Proceedings of the National Academy of Sciences. 2021 Jun 22;118(25):e2023286118.
9. Schirrmeister RT, Springenberg JT, Fiederer LDJ, Glasstetter M, Eggensperger K, Tangermann M, et al. Deep learning with convolutional neural networks for EEG decoding and visualization. Human Brain Mapping. 2017;38(11):5391-420.